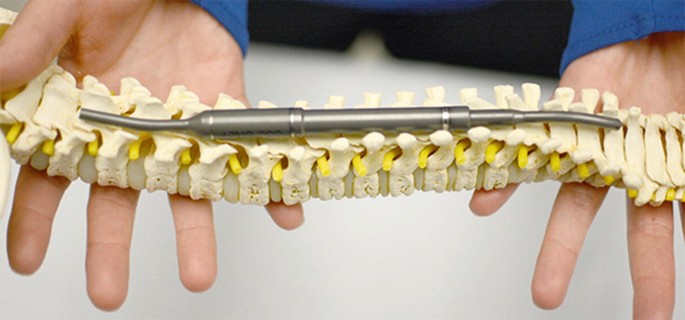
Spinal implants are engineered to support and stabilize one of the most complex and mechanically demanding structures in the human body. Over time, these devices are exposed to repetitive loads, stress and micro-movements that can influence their durability and performance. Dr. Larry Davidson, an experienced specialist in spinal care, recognizes that understanding material fatigue is essential not only for improving implant longevity but also for optimizing patient safety and outcomes over the long term.
Material fatigue refers to the gradual deterioration of an implant’s structural integrity due to repeated mechanical stress. While spinal implants are designed to be strong and biocompatible, long-term studies are now providing clearer insights into how they behave years after surgery. These findings are shaping everything from material selection and surgical techniques to postoperative care and device monitoring.
The Mechanics Behind Material Fatigue
When an implant is subjected to cyclic loading, such as bending, torsion and compression from daily activities, the internal structure of the material can begin to weaken. Even small, repeated forces can create micro-cracks that expand over time. Eventually, this can lead to partial failure, loss of structural integrity or, in rare cases, catastrophic implant breakage.
Spinal fusion hardware, such as rods, screws and cages, bears the brunt of spinal motion until bone fusion is complete. If the fusion process is delayed or incomplete, implants remain under stress longer than intended. This prolonged mechanical demand can accelerate material fatigue, especially in areas of high motion or load, such as the lumbar spine.
Insights from Long-Term Clinical Studies
Longitudinal studies have helped illuminate the true performance of spinal implants over five, ten or even twenty years. These investigations track implant survival rates, revision surgery frequencies and failure modes across diverse patient populations and surgical approaches.
One study published in the Spine Journal followed patients who underwent lumbar fusion with titanium rods and pedicle screws. After a ten-year follow-up, researchers found that while mechanical failure was relatively uncommon, it was more likely in cases where fusion was incomplete, or the hardware was subjected to abnormal stress due to poor alignment or osteoporotic bone quality.
Another multicenter review examined the fatigue life of interbody cages under cyclic loading in simulated conditions. The results revealed that PEEK cages maintained structural integrity longer than expected, but their performance was sensitive to the thickness and spacing of their lattice designs.
Materials Matter: Titanium, PEEK and Beyond
Titanium and PEEK remain the most used materials in spinal implants. Titanium offers excellent strength and biocompatibility, with a high resistance to fatigue. However, its stiffness can lead to stress shielding, where the bone absorbs less load and thus remodels more slowly or insufficiently. In long-term scenarios, this may indirectly contribute to mechanical strain on the hardware.
PEEK, a high-performance polymer, provides a more bone-like modulus of elasticity and creates fewer imaging artifacts on MRI or CT. However, it may be more susceptible to micro-fractures or fatigue wear, particularly when used in thinner, more delicate constructs.
Emerging materials such as carbon fiber-reinforced polymers and additive-manufactured porous metals are showing promise in mitigating fatigue risks. These materials offer a blend of strength, flexibility and bone integration properties that may improve implant performance under long-term loading conditions.
Surgical Technique and Implant Fatigue
The way an implant is inserted plays a critical role in how it performs over time. Misalignment during rod bending, overtightening of pedicle screws or insufficient endplate preparation can all introduce stress points that accelerate fatigue. Fusion techniques that fail to achieve solid union within the expected healing window can also increase the burden on implants, leading to early wear.
Minimally invasive approaches, while beneficial for soft tissue preservation, may place additional stress on narrower, lower-profile hardware. As a result, surgeons must weigh the trade-offs between surgical access and long-term implant durability when planning procedures.
Monitoring and Diagnosing Implant Fatigue
Detecting material fatigue in its early stages is difficult, as symptoms may mimic other complications such as nonunion or adjacent segment disease. Patients might report persistent pain, reduced mobility or neurologic symptoms, all of which warrant thorough evaluation. Advanced imaging techniques, including dynamic radiographs, CT scans and MRI, can help identify signs of fatigue, such as micro-motion at the implant interface, subsidence or breakage.
In some cases, exploratory surgery may be required to assess the structural integrity of the hardware. Wearable health technology may eventually monitor implant performance. Future implants embedded with sensors could track load distribution and provide early warnings about unusual stress patterns or potential failures, enabling preemptive interventions.
Dr. Larry Davidson mentions, “Emerging minimally spinal surgical techniques have certainly changed the way that we are able to perform various types of spinal fusions. All of these innovations are aimed at allowing for an improved patient outcome and overall experience.” As these techniques evolve alongside sensor-integrated implants, surgeons may soon gain real-time insights into implant function, bridging the gap between surgical precision and long-term postoperative care.
What Patients Should Know
Patients undergoing spinal fusion should be made aware that while implant fatigue is rare, it is a potential long-term risk. This risk increases in cases of poor bone quality, high physical activity or incomplete fusion. Regular follow-up appointments and imaging can help catch early signs of fatigue-related complications before they require major intervention.
Patients should also be educated on the importance of proper biomechanics post-surgery; this includes maintaining a healthy weight, engaging in core-strengthening exercises and avoiding activities that place excessive strain on the spine during the healing process.
Transparent conversations about implant materials, expected lifespan and follow-up care can foster more realistic expectations and empower patients to take an active role in preserving the integrity of their spinal implants.
Pushing the Boundaries of Durability
Long-term studies on material fatigue are driving meaningful changes in the way spinal implants are designed, selected and monitored. Surgeons are using this data to refine their techniques, choose materials more strategically and advocate for long-term follow-up that goes beyond immediate postoperative success.
Research is ongoing to develop fatigue-resistant implants that are not only structurally superior but also smarter and capable of adapting to a patient’s unique loading patterns and activity levels. Innovations in material science, design engineering and patient-specific planning are aligning to extend the life and safety of spinal hardware. These insights into material fatigue are reshaping the future of spinal care, encouraging a deeper commitment to both biomechanical precision and lifelong patient well-being.